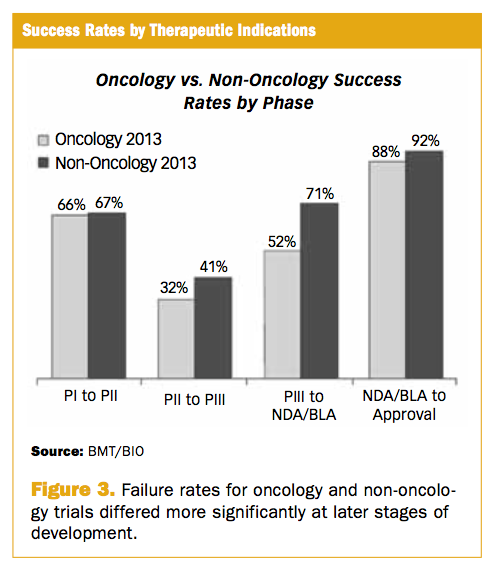
When one or more Phase 3 trials are completed the researchers examine the results and decide whether the drug has demonstrated effectiveness and an acceptable safety profile in treating a disease. A clinical trial to explore the clinical use of a new drug esp.
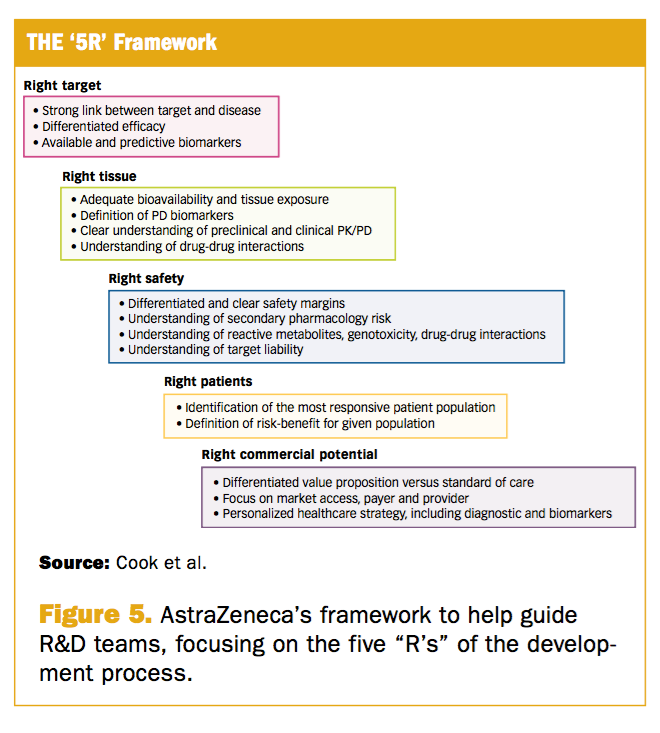
Phase Iii Trial Failures Costly But Preventable
Phase 3 drug trials are typically double-blind meaning that neither the patients nor the researchers know which group is receiving the new treatment or the existing standard.
Phase 3 trial meaning. The most reliable study design is a randomized blinded and placebo- or active-controlled trial to verify that the results are beneficial. Phase III Clinical Trial Phase III studies are done to study the efficacy of an intervention in large groups of trial participants from several hundred to several thousand by comparing the intervention to other standard or experimental interventions or to non-interventional standard care. In the United States when phase III clinical trials or sometimes phase II trials show a new drug is more effective or safer than the current treatment a new drug application NDA is submitted to the Food and Drug Administration FDA for approval.
Phase III of a clinical trial usually involves up to 3000 participants who have the condition that the new medication is meant to treat. Phase 3 clinical trials are much larger than the ones conducted in Phase 1 or Phase 2. Phase 3b Clinical Trial means a human clinical trial of the Licensed Product that a is voluntarily conducted after the filing with the FDA of the NDA for the Licensed Product but prior to obtaining Regulatory Approval of such NDA and b is not required or requested by the FDA as a condition of or in connection with obtaining such Regulatory Approval.
Phase 3 is therefore a vital phase of drug development and billions may be spent progressing the drug to a phase 3 trial only for the drug to prove ineffective in. While safety remains a focus these trials are primarily. 2012 Farlex Inc.
No placebos are given. Phase 3 trials are the pivotal final trials before a vaccine is approved for widespread use. A subcategory of a Phase-3 clinical trial performed near the time of approval to accumulate additional findings which and may be required as a condition of regulatory authority approval.
Phase 3 trial. Trials in this phase can last for several years. These trials often include thousands of enrollee patients.
The Phase 3 trial is an international study that aims to evaluate the safety and efficacy of elafibranor 120mg given once daily in patients with NASH and fibrosis. Before phase III the substance is not regarded as a drug but after a positive phase III trial it becomes a drug. These trials involve hundreds or even thousands of participants and are often conducted in many regions around the globe.
The main objective of phase III trials is to verify the therapeutic action of a new substance in a large number of patients essentially to determine the riskbenefit ratio. If so the company can submit a New Drug Application NDA which contains all of the data and information gathered at every stage of the process through the results of the Phase 3. Dossier review may continue while associated Phase-3B trials are conducted.
Relative to other known effective agents the current standard of care. Treatments that have been shown to work in phase 2 are evaluated again and on a larger scale prior to being approved for general use. By law all such patients receive real treatment.
For clarity a trial called a Phase 23 trial shall be considered a Phase 3 trial if it satisfies the requirements of 21 CFR. Usually two or more phase 3 trials are conducted. Phase 3 Trial means a human clinical trial of a Product in any country that would satisfy the requirements of 21 CFR.
Phase 3 clinical trials are designed to test whether the investigative treatment is better than the standard clinical method for the targeted health condition. The FDA reviews the results from the clinical trials and other relevant information.
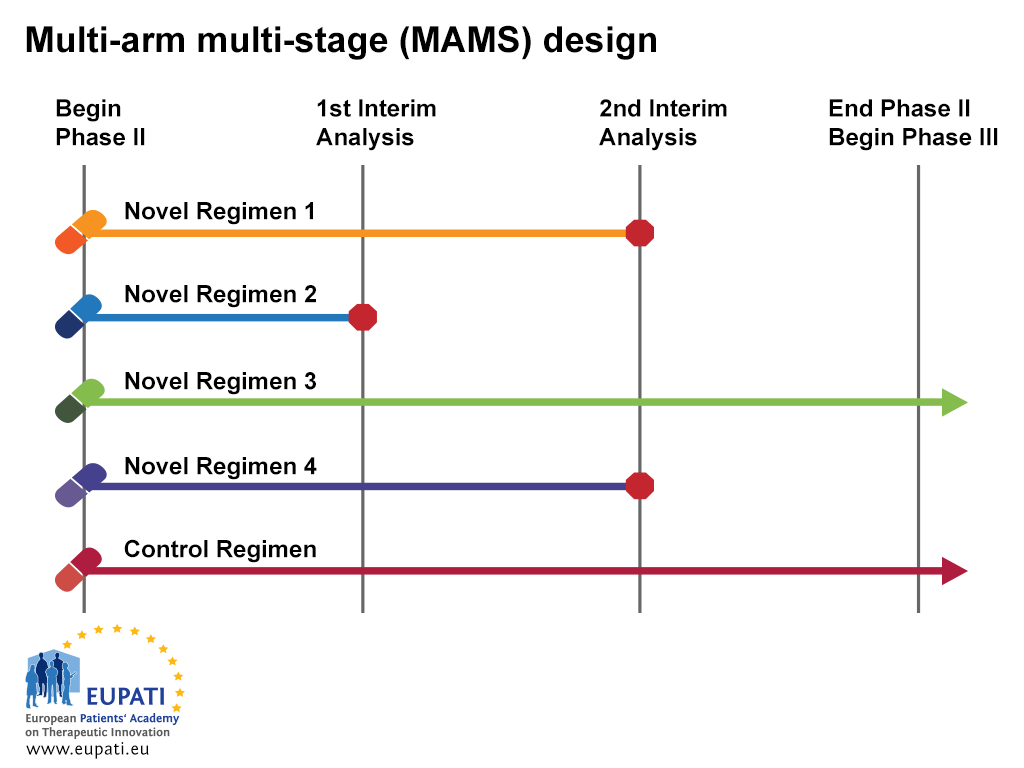
New Approaches To Clinical Trials Adaptive Designs Eupati Toolbox
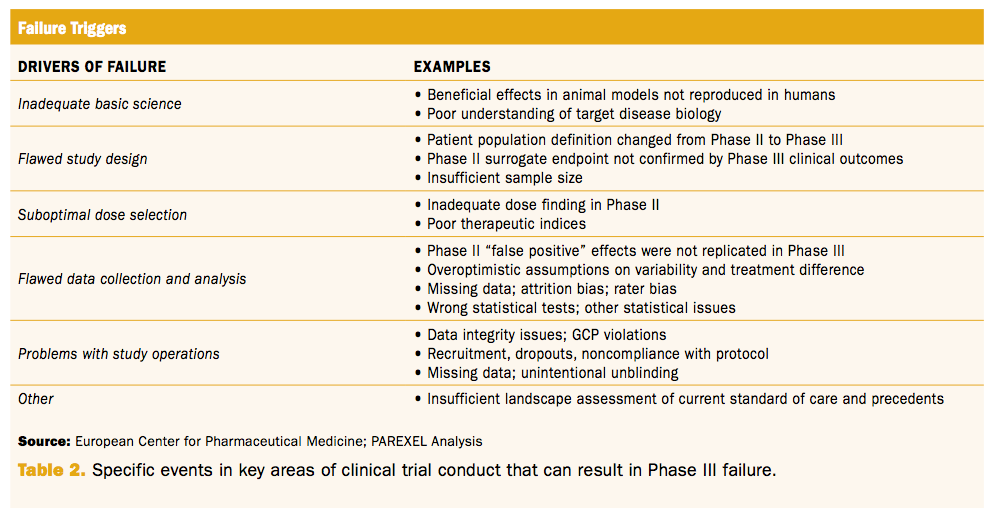
Phase Iii Trial Failures Costly But Preventable
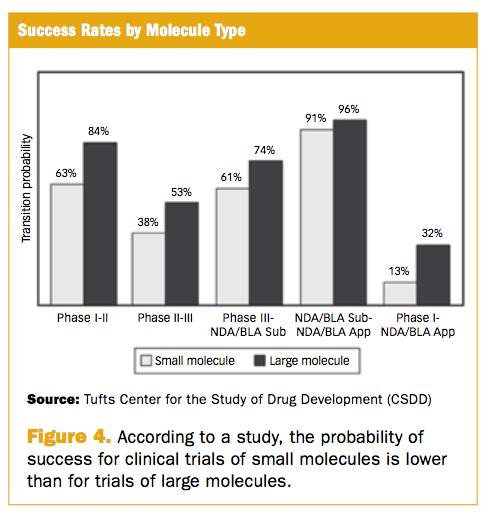
Phase Iii Trial Failures Costly But Preventable

Clinical Trial Medicine Britannica
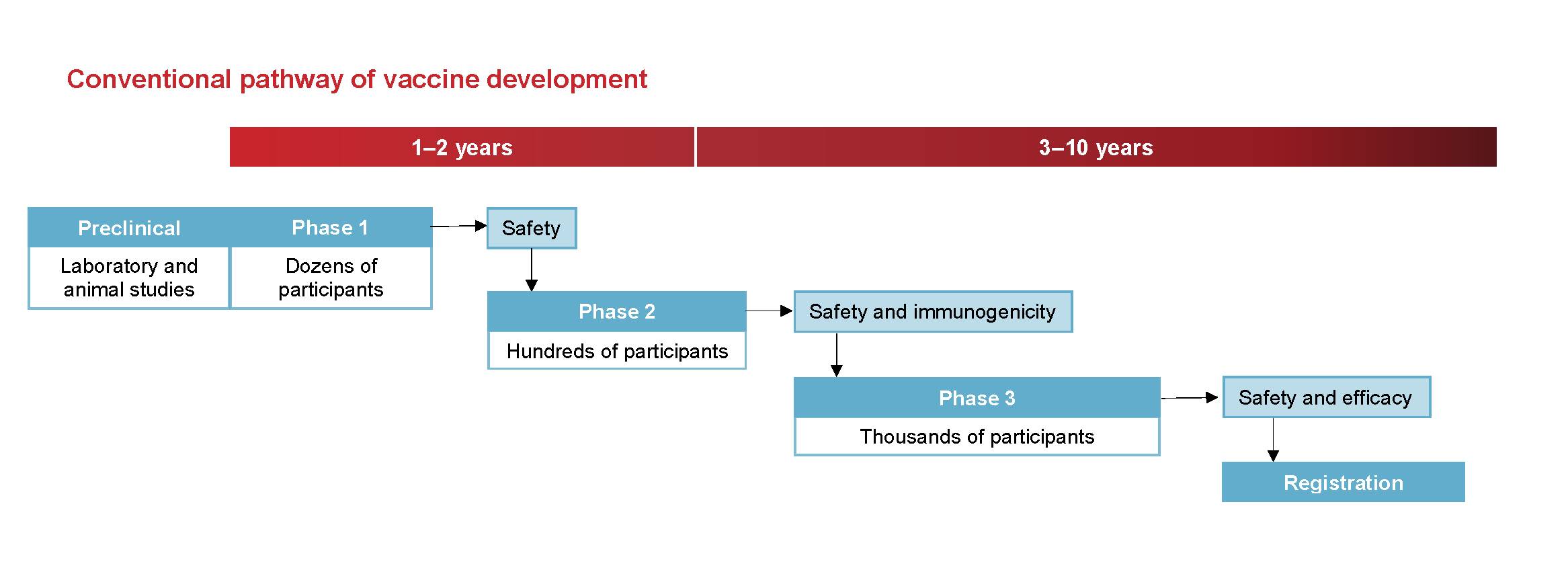
Phases Of Clinical Trials Ncirs
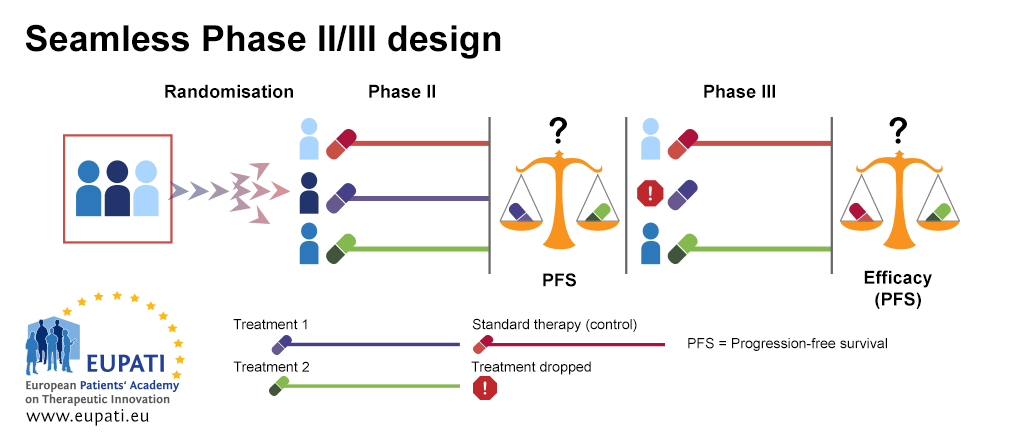
New Approaches To Clinical Trials Adaptive Designs Eupati Toolbox
Phase Iii Trial Failures Costly But Preventable

Understanding Clinical Trial Terminology What S A Phase 1 2 Or 3 Clinical Trial Concert Pharmaceuticals
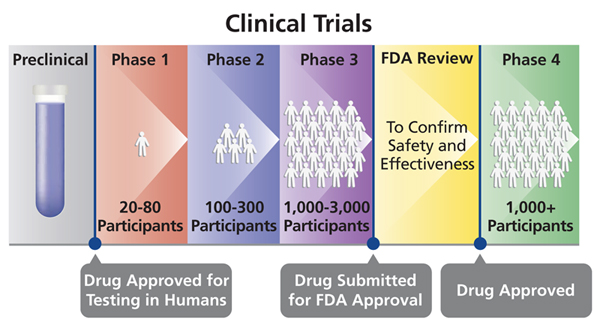
First In Human Covid 19 Vaccines Tales Of Phase 1 Clinical Trials Past Absolutely Maybe
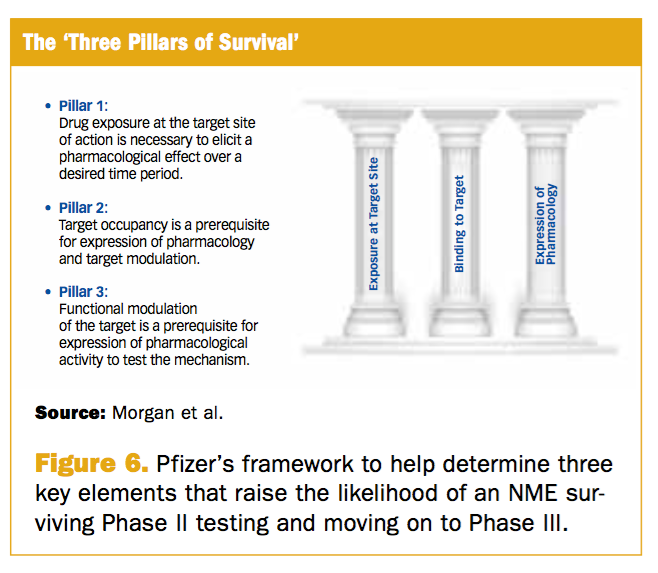
Phase Iii Trial Failures Costly But Preventable
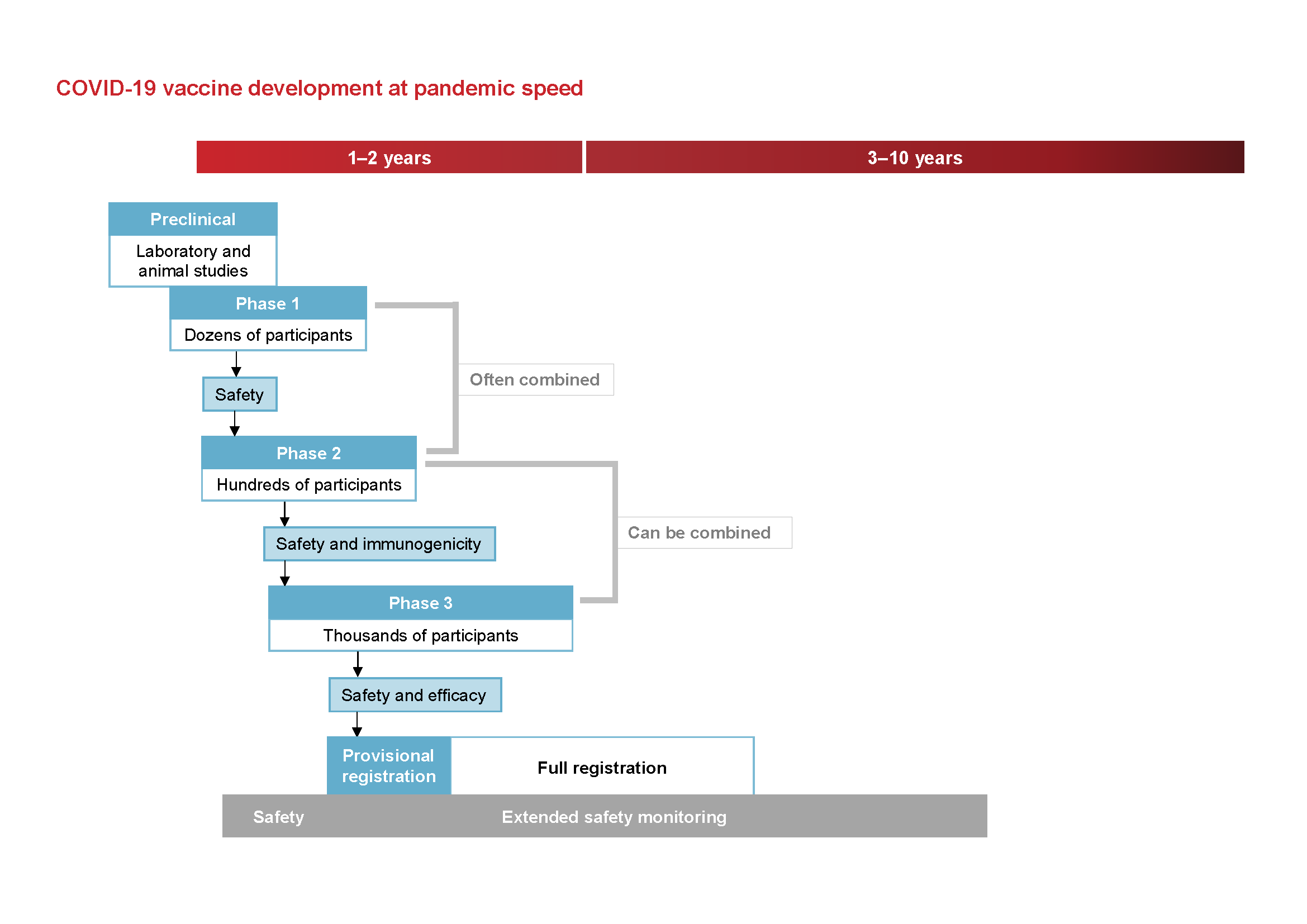
Phases Of Clinical Trials Ncirs
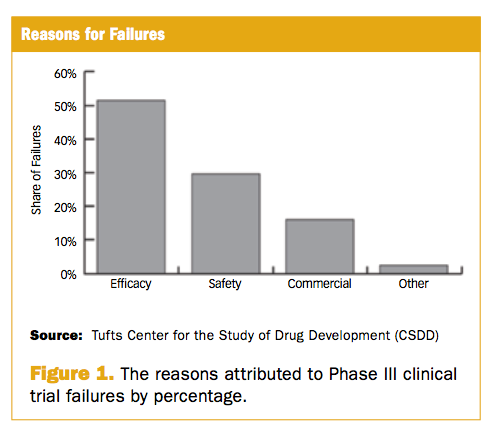
Phase Iii Trial Failures Costly But Preventable
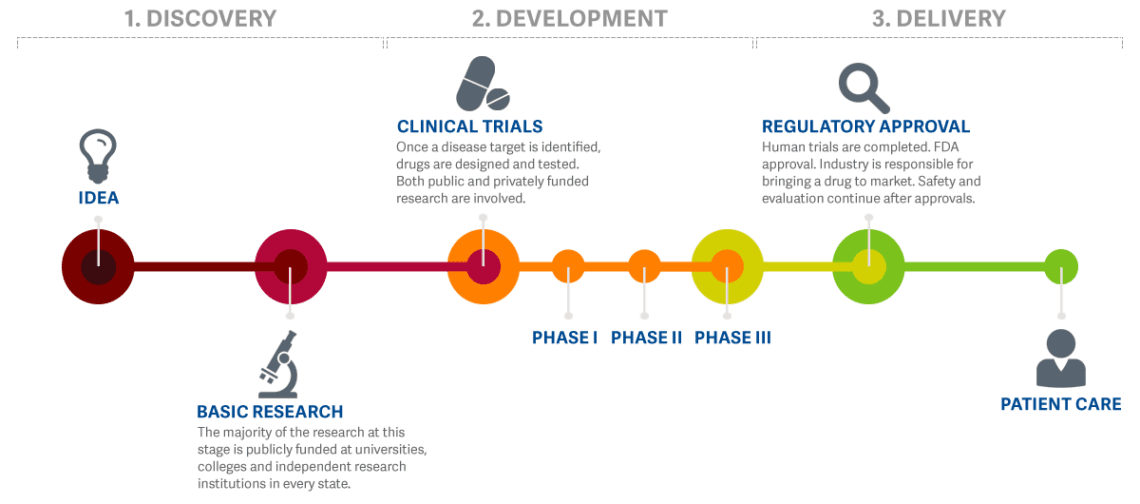
Clinical Trials Gbs Cidp Foundation International

Covid 19 Vaccine Race Month 6 First Emergency Use Phase 3 Trials Absolutely Maybe